DIRECTOR
Iowa Laboratory Appraisal Program-CLIA
Since 2002, the Hygienic Laboratory has been responsible for the administrative oversight of the CLIA (Clinical Laboratory Improvement Amendments) laboratory program in Iowa, and serves as the state agency representative for the Center for Medicare and Medicaid Services CLIA program.
CLIA regulations establish quality standards for laboratory testing performed on specimens from humans – such as blood, body fluids and tissue – for the purpose of diagnosis, prevention, or treatment of disease, or assessment of health. Under a contract with the Iowa Department of Inspections and Appeals, the Hygienic Laboratory provides personnel to conduct these surveys.
Federal law requires any laboratory or facility performing laboratory tests of human specimens for the purpose of diagnosis, prevention or treatment of disease to meet certain requirements, including a CLIA certification. Every two years, laboratories with certificates of compliance or accreditation are subject to routine inspections, referred to as surveys.
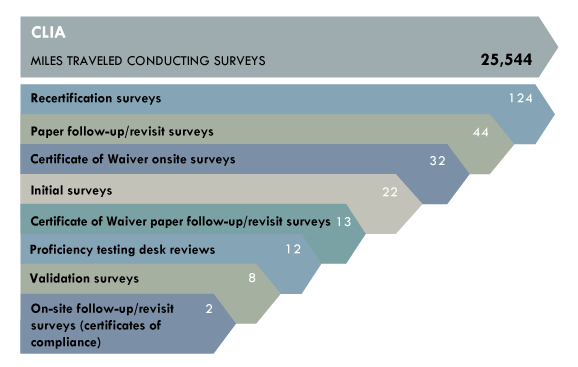
Major achievements:
- Conducted 32 on-site surveys and 16 follow-up/revisit surveys of facilities with a Certificate of Waiver for fiscal year 2014. These surveys were in addition to more than 150 routine on-site surveys that must be conducted for the fiscal year.