
| May 2014 | |||||||||||
| Top stories | |||||||||||
| In the news | |||||||||||
| Photos | |||||||||||
| Contact us | |||||||||||
| Archive | |||||||||||
|
Clinical evaluation of suspected MERS |
The State Hygienic Laboratory worked in conjunction with the Iowa Department of Public Health and the Centers for Disease Control and Prevention to create a protocol for clinical evaluation of suspected MERS cases. Additional information is available on the State Hygienic Laboratory website.
What is MERS?
Middle East Respiratory Syndrome (MERS) is a viral respiratory illness first reported in Saudi Arabia in 2012. It is caused by a coronavirus called MERS-CoV. Most people who have had confirmed MERS-CoV infection developed severe acute respiratory illness. Their symptoms included fever, cough and shortness of breath. About 30 percent of these people died.
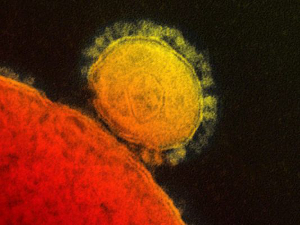
While most cases have been linked to four countries in or near the Arabian Peninsula, the first U.S. imported cases of MERS were confirmed earlier this month on May 2 and May 11. The two U.S. cases were not linked. After completing additional and more definitive laboratory tests, CDC officials have concluded that the May 2 MERS case confirmed in an Indiana patient did not spread the virus to an Illinois associate during a business meeting they had before the patient became ill and was hospitalized. This underscores indications that transmission of reported MERS cases has occurred through prolonged exposures of health care workers and patients, or between care providers and patients.
This virus has spread from ill people to others through close contact. However, the virus has not shown to spread in a sustained way in communities. The situation is still evolving. More information is available through the Centers for Disease Control and Prevention .
How are patients and suspected cases evaluated?
Clinicians and health care professionals should immediately report patients with suspect MERS-CoV infection to the Iowa Department of Public Health (IDPH) at 800-362-2736. Testing at the State Hygienic Laboratory will be arranged, if indicated.
The CDC provides the following evaluation guidance for clinicians and health care providers:
A patient under investigation (PUI) is a person with the following characteristics:
- Fever (≥38°C, 100.4°F) and pneumonia or acute respiratory distress syndrome (based on clinical or radiological evidence); and either
- history of travel from countries in or near the Arabian Peninsula within 14 days before symptom onset; or
- close contact with a symptomatic traveler who developed fever and acute respiratory illness (not necessarily pneumonia) within 14 days after traveling from countries in or near the Arabian Peninsula; or
- is a member of a cluster of patients with severe acute respiratory illness (e.g. fever and pneumonia requiring hospitalization) of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
What are the next steps if testing is approved by IDPH?
Specimen Collection:
If testing is approved by IDPH, contact the State Hygienic Laboratory at 319-335-4500 prior to collection of a specimen for full instructions.
Kits and Test Request Forms:
To order “Viral Detection and Viral and Bacterial PCR” collection kits and test request forms, contact the Hygienic Lab at 319-335-4500 or fill out the Order Clinical Kits Form.
If testing is approved by IDPH, please fill out the “Viral Detection and Viral and Bacterial PCR” test request formand write in “MERS-CoV approved by IDPH” for test requested.
Testing
The Food and Drug Administration has allowed use of the "CDC Novel Coronavirus 2012 Real-time RT-PCR Assay" under an Emergency Use Authorization.

Anna Yakos, public health microbiologist, preps the MERS-CoV CDC assessment panel for extraction on the Biomerieux NucliSENS easyMAG extraction platform.
- This test has not been FDA cleared or approved;
- This test has been authorized by FDA under an Emergency Use Authorization for use by qualified laboratories;
- This test has been authorized only for the detection of MERS-CoV and not for any other viruses or pathogens; and
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostics for detection of MERS-CoV under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1).
More information including fact sheets for patients and physicians can be found at the FDA