
The Iowa Newborn Screening Program identifies infants at risk for more than 49 inherited diseases by testing drops of blood obtained from a simple heel stick shortly after birth. Most newborns with an inherited condition show no obvious signs of disease. However, with special tests, the Iowa Newborn Screening Program can identify an infant who may be at risk, and alert the doctor and caregivers of the need for immediate, sometimes critical, medical treatment.
A nationally recognized leader in newborn screening, the Iowa program is administered by the Iowa Department of Public Health, which partners with the State Hygienic Laboratory and the University of Iowa Stead Family Children’s Hospital.
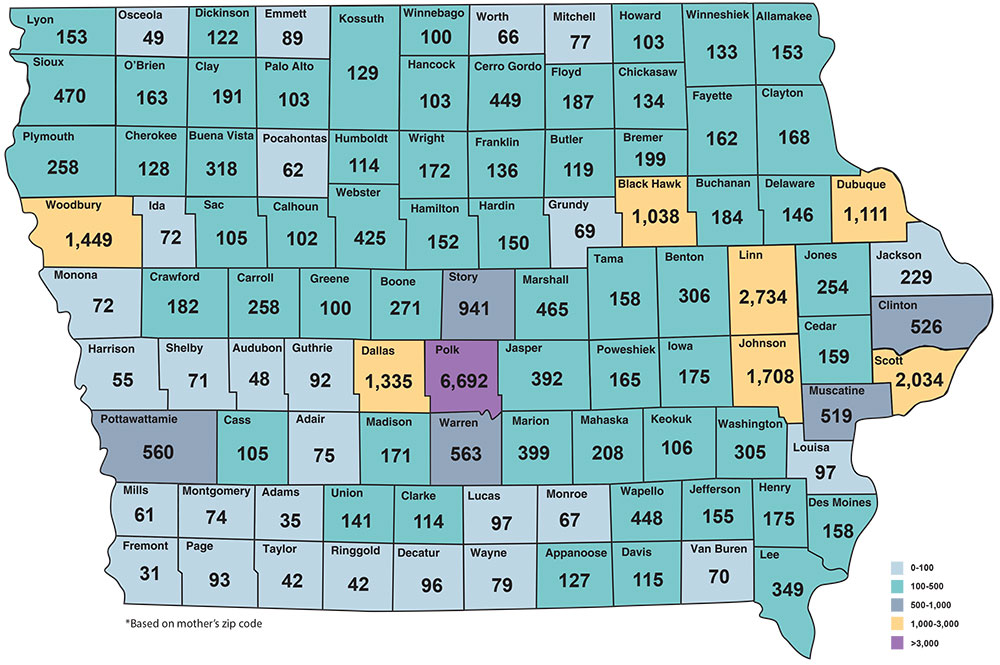
Babies Screened in Iowa FY2017 = 35,012*
SCREENING VOLUME
- Iowa: 323,628
- North Dakota: 107,610
- South Dakota: 108,371
TOTAL: 539, 609
SPECIMENS RECEIVED
- Iowa: 40,532
- North Dakota: 13,471
- South Dakota: 13,571
TOTAL: 67, 574
HIGHLIGHTS
- In September 2016, the Iowa Newborn Screening Program celebrated its 50th anniversary. Iowa Gov. Terry Branstad signed a proclamation recognizing the anniversary and declaring September as Newborn Screening Awareness Month.
- Screening for Severe Combined Immunodeficiency (SCID) was added in July 2016 to the conditions that SHL screens for on behalf of North Dakota. (The rare condition was added to the Iowa screening panel in 2014 and to the screening done on behalf of South Dakota in 2015.) SCID represents a fundamental change in the testing methodology compared to the other conditions on the screening panel. It is the first condition to directly use a DNA target as the marker for the disorder, and the first condition for which a cure is available.
- The Iowa Newborn Screening Program continued its work as a part of the Association of Public Health Laboratory’s (APHL) NewSTEPs pilot Collaborative Improvement and Innovation Network (CoIIN) for Timeliness in Newborn Screening. The goal of this ongoing project is to achieve timely reporting of results in 95 percent of newborns that receive driedblood spot (DBS) newborn screening. The Iowa CoIIN project is working to increase awareness of the newborn screening process and provide education for best practices with the intent of improving response time for babies born in Iowa, and eventually for all babies born in the U.S. In FY2017, the Iowa Newborn Screening Program achieved its goal of receipt of 95 percent of newborn screening specimens within 65 hours of birth.
- New versions of blood spot collection forms were created and distributed in Iowa, North Dakota and South Dakota. Educational webinars on the use of these forms were provided to hospital staff. An educational resource flip chart, “How to Complete the Iowa Newborn Screening Cards,” was created for Iowa and North Dakota and distributed to all hospitals and clinics in Iowa.
- The Iowa newborn screening laboratory validated a new cystic fibrosis mutation test method in the span of three months.
- New instrumentation for the punching of dried blood spots from the filter paper specimens went online. The new punching instrumentation will help provide more accurate identification of specimens, help reduce lab errors and enhance traceability through the analytical process.
- A comprehensive site review was conducted by the APHL NewSTEPs (Newborn Screening Technical Assistance and Evaluation Program) team of experts who assessed various components of our newborn screening program, including the laboratory system, birth facilities and follow-up system.
- Continued research collaboration on developing models predictive of gestational age using newborn screening biomarkers with Kelli Ryckman, associate professor in the University of Iowa Department of Epidemiology. SHL involvement in this project involved testing nearly 1,000 research specimens from Africa and India for more than 70 newborn screening biomarkers.
- Awarded a grant by APHL to host a “Deliberative Community Engagement” event with Iowa citizens as part of a review of new conditions for Iowa’s screening panel.



